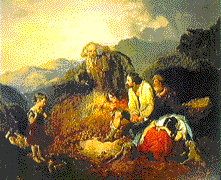|
Most plant-parasitic
nematodes live in the soil and infect plant roots. The cyst nematodes
(Heterodera and Globodera species) and root-knot nematodes (Meloidogyne
species) are the most damaging groups of plant-parasitic nematodes,
and they have evolved very complex interactions with their host
plants. As with all nematodes, cyst and root-knot nematodes grow
by a series of four molts to the adult stage. Worm-shaped juveniles
at the second life stage (J2) penetrate plant roots completely.
The nematodes migrate to the center of the roots, where they must
transform selected plant root cells into an elaborate feeding
site to support the further growth and reproduction of the nematode.
Every plant-parasitic
nematode (see Figure 2) has a "stylet" (a hypodermic needle-like
feeding spear in the nematode head) that they use to withdraw
nutrients from plant cells and also to secrete substances into
the plant. The substances secreted by the nematodes allow them
to penetrate and migrate within the roots and to transform the
plant cells into feeding sites. Dr. Davis' lab is beginning to
understand this process at a molecular level-with the goal of
interfering in successful nematode parasitism to prevent crop
damage. Some of the discoveries have potential commercial application
to prevent nematode damage of plants, and two of the patent disclosures
from the Davis lab are described here.
|
 |
|
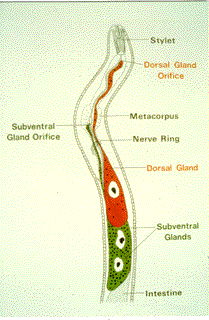
Figure
2: Illustration of an infective juvenile of the soybean cyst
nematode, Heterodera glycines, showing the (hypodermic needle-like)
"stylet" in the nematode head that it uses to feed from plant
cells. Substances produced in the (red and green) esophageal
gland cells of the nematode are secreted out through the stylet
into plant root tissue.
|
|
|
|
|
|
Novel Cellulases
NCSU
Patent Disclosure Number 99-28
Figure 3 shows a
soybean cyst nematode (SCN) juvenile (Heterodera glycines) that
had been induced to secrete substances (stained blue) from its
stylet. Since the nematodes are extremely small, it is almost
impossible to collect enough of the secretions for direct analysis.
Instead, the Davis lab has collaborated with other scientists
to clone the nematode genes that produce the secretions. Once
the genes are cloned, the analysis becomes much easier. One of
the SCN genes cloned encodes an enzyme called a cellulase (b-1,4-endoglucanase)
that the nematode secretes from its stylet to degrade the cellulose
walls around plant cells. This turned out to be the first cellulase
gene ever cloned from any animal-cellulase genes had only previously
been cloned from bacteria, fungi, and plants. This discovery was
published in 1998 by Davis and his collaborators (Smant, et al.)
in the Proceedings of the National Academy of Sciences. A year
later, the Davis lab was able to show that the juveniles of SCN
secrete the cellulase to allow the nematodes to penetrate and
migrate in plant roots. This discovery was highlighted on the
cover of Molecular Plant-Microbe Interactions (Figure 4), which
shows a bright green fluorescence that indicates where the nematode
has secreted its cellulase through its stylet into the plant root.
Inhibition of the nematode cellulase activity could represent
a commercial way to prevent cyst nematode parasitism of crop plants,
and this is a primary reason why an application to protect the
discovery of the novel nematode cellulases has been pursued by
NC State University.
Endoglucanase
Gene Promoter Specifically Upregulated by the Root-Knot Nematode
NCSU
Patent Disclosure Number 00-74
|
|
|
|

|
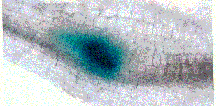
Figure
3: Production of secretions (stained blue) out the stylet of an
infective juvenile of the soybean cyst nematode, Heterodera glycines.
The secreted substances allow the nematode to penetrate and feed
from plant roots.
|
|
Figure
4: A journal cover showing the secretion of an enzyme (bright green
fluorescence) called cellulase (b-1,4-endoglucanase) by an infective
soybean cyst nematode juvenile as it penetrates a plant root. The
cellulase enzyme degrades plant cell walls and allows the nematode
to invade the plant root. Inhibition of nematode cellulase activity
could be a commercial application to reduce nematode damage to crop
plants.
|
 |
| |
|
 |
|
Figure
5: Juvenile stages of nematodes (stained red) that have entered
a plant root and started to feed. The nematode on the left
has begun to swell as it feeds from the same plant cells and
progresses through several molts to the swollen and immobile
adult (reproductive) life stage.
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
 |
|
Figure
7: The blue color demonstrates a plant cellulase gene that
has been "turned-on" (upregulated) in the infection site of
a root-knot nematode. The swollen portion of the root (called
a gall) is a typical symptom of plant root infection by root-knot
nematodes (as observed by many unfortunate home gardeners).
|
| |
|
|
| |
|
|
| |
|
The
secretions of nematodes also induce many changes in the plant cells
that the nematodes use for feeding. The root-knot nematodes (Meloidogyne
species) are similar to cyst nematodes in that the worm-shaped juveniles
enter plant roots completely and then begin to feed (Figure 5).
The nematodes transform selected plant cells around their head into
"giant-cells" that are much larger than normal plant cells and have
multiple nuclei (Figure 6). The nematodes require the giant-cells
to feed as they become swollen and immobile at the adult life stages.
Many plant genes are "turned-on" (upregulated) in the giant-cells
to make the feeding site for the nematode. A Ph.D. student (Melissa
Goellner) in the Davis lab discovered that the root-knot nematodes
upregulate a cellulase (endoglucanase) gene of plant origin specifically
within the giant-cells. Goellner obtained plants from Oded Shoseyov
at the Hebrew University of Jerusalem that were bioengineered to
produce a blue color in any plant cells that expressed the (cel
1) plant cellulase gene. Goellner discovered that the cellulase
gene (blue color) appeared to be specifically upregulated at the
site of nematode infection (Figure 7). She observed under a microscope
that the plant cellulase gene (blue color) was only expressed in
the giant-cells that the root-knot nematode had induced for feeding
within the plant root Figure 8). Because the plant cellulase gene
is upregulated by nematode feeding, the portion of the plant cellulase
gene that responds to the nematode (the "promoter") could be used
to express "anti-nematode" genes when the nematode attempts its
normal feeding. This discovery may have tremendous commercial applications
for bioengineering crop plants with a novel form of resistance to
root-knot nematodes, and this is one reason why an application to
protect the discovery of this plant cellulase promoter response
to nematode parasitism has been pursued by N.C. State University.
|
 |
|
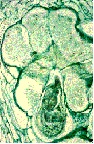
Figure
6: Section of a plant root observed under a microscope that
shows six "giant-cells" surrounding a swollen root-knot nematode
(Meloidogyne species). The nematode transformed these plant
cells into these giant cells for feeding. The expression of
plant genes within the many nuclei (red dots) of the giant
|
| |
| |
| |
| |
| |
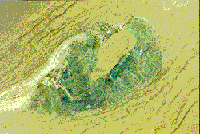 |
Figure 8:
Section of a plant root observed under a microscope that shows plant
cellulase gene expression (blue color) exclusively within the giant-cells
surrounding a swollen (clear) root-knot nematode (Meloidogyne species).
This plant cellulase gene may be modified to produce an "anti-nematode"
product when the nematode feeds, which could have commercial applications
to prevent crop damage by root-knot nematodes.
|
|
Dr.
Jean Beagle Ristaino
PCR Assays
for Phytophthora Species
U.S.
Patent Number 5,780,271
|
 Click
to enlarge image
Click
to enlarge image
|
Dr. Jean Beagle Ristaino
is a professor in the Department of Plant Pathology at NC State University.
Her patent, "PCR [Polymerase Chain Reaction] assays for phytophthora
species," was issued under U.S. Patent Number 5,780,271 in 1998. This
invention is a method of screening for the presence of pathogens in
potatoes, tomatoes and other plant species using oligonucleotide primers.
Her research is being used to determine the source of the potato late-blight
pathogen, Phytophthora infestans that infected the Irish potato and
caused the great Irish potato famine of the 1840s. The research is also
being used to shed light on the origin of the modern form of the late-blight
pathogen.
Windows
to the Past:
Tracking
19th-century Irish potato famine epidemics using herbarium
specimens
|
Contributed
by Dr. Jean Beagle Ristaino, Professor, Department of
Plant Pathology, NC State University, Raleigh, N.C.
|
|
|
|
|
|
More
than 150 years ago, the late-blight pathogen Phytophthora infestans
struck the Irish potato crop, leading to famine. Epidemics first
began in North America in 1843 and in late-summer of 1845 and swept
across Europe to Ireland, destroying potato crops in its wake. In
its aftermath, more than one million people died and another two
million emigrated from Ireland. As depicted in this painting by
Daniel MacDonald, "The Discovery of Potato Blight," c. 1852, the
Irish people were dependent on potatoes as a sole food source, and
when the crop failed millions suffered the consequences.
|
|
|
Late-Blight
in Sampson County, North Carolina, 1998
Late-blight has
become a reemerging disease on both potato and tomato crops in
the United States and many other areas of the developed and developing
word because of widespread occurrence of new genotypes of the
pathogen that are highly resistant to the commonly applied fungicide
metalaxyl. Most isolates of the pathogen found worldwide are not
resistant to this fungicide. This potato field in Sampson County,
North Carolina was destroyed by the pathogen in a matter of weeks.
|
|
|
|
Sporangia
of the Late-Blight Pathogen
|
 |
|
(Slide
courtesy of W. Fry, Cornell University)
|
| |
| |
| |
| |
| |
| |
|
Late-Blight
Infected Potato Tuber
|
| |
|
|
Phytophthora
infestans
|
The late-blight
pathogen produces asexual spores known as sporangia. The microscopic,
lemon-shaped sporangia are borne on tiny branched stalks called
sporangiosphores. Sporangia are easily dislodged from the sporangiophores
and spread by wind and rain.
|
|

|
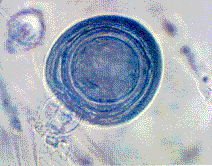
Oospore
Some
of the fungicide resistant isolates of Phytophthora infestans
are not only surviving but also reproducing sexually and forming
overwintering, thick-walled oospores in the presence of a number
of commonly used fungicides on potatoes. This clever pathogen
has adapted well to the pesticides that have been developed
to manage it. Clearly, new and innovative research on the biology
of the pathogen is needed to manage effectively late-blight
epidemics.
|
|
 |
 |
Leaf
Lesion on Potato
Caused
by Phytophthora infestans
Sporangia infect leaves
of potatoes or tomatoes and cause grayish black lesions. The pathogen
then produces millions of additional sporangia in white tufts on
the leaves, stems, tubers, or fruit that can be easily spread to
the next susceptible plant.
|
|
Herbarium
materials are being used in the Laboratory of Dr. Jean Ristaino
(shown on right) at North Carolina State University with the
help of Dr. Carol Trout Groves (shown on left), USDA, Orono,
Maine, and research specialist Greg Parra to help clarify
present-day questions about the biology of the late-blight
disease. The tools of molecular biology coupled with herbarium
specimens infected with Phytophthora infestans offer a unique
approach to addressing questions concerning the nature and
source of populations of old epidemics and the migration of
the pathogen worldwide. "We are most interested in identifying
the actual genotype of the pathogen that caused the 19th-century
Irish, European and United States epidemics.
|
|
|
|
It has
been proposed that a particular genotype of Phytophthora
infestans referred to as the US-1 genotype, was the
genotype that caused the original epidemics during
the famine in Ireland. The previous studies were based
on present-day collections of the pathogen. No one
has ever tested actual specimens from the 19th-century
epidemics to confirm or refute our current hypotheses.
We plan to track historical migration patterns of
the devastating potato pathogen using 19th-century
dried potato leaves and identify the genotype(s) responsible
in our work funded in part by a grant from the Committee
for Research and Exploration of the National Geographic
Society."
|
|
|

Infected
Dried Modern Leaf and Herbarium Sheet
|
What
are the unsolved mysteries of potato late-blight? There are
many questions that remain to be answered concerning the origins
of the late-blight pathogen, Phytophthora infestans. Some
scientists ascribe to the Mexican theory believing that the
pathogen originated in Mexico, which is clearly a present-day
center of diversity of the pathogen. Other scientists ascribe
to the Peruvian theory that the pathogen evolved in South
America, the ancestral home of the potato. In fact, the first
potato cultivatars to succumb to the disease in Europe in
the 1840s included South American types such as "Lima," "Cordilleres,"
and "Peruviennes." Bat guano was used as fertilizer on potatoes
and imported from Peru during the 1840s. The development of
steamships also led to greater export of potato tubers from
Peru to European countries in the 1840s. A third group of
scientists has suggested that the pathogen originated in Mexico
but inoculum for the 19th-century epidemics came from Peru.
Development of a clear understanding of the origins of the
pathogen will be useful as present-day potato and tomato breeders
search for sources of host plant resistance to the disease.
|
| |
Dr.
John Lindley collected this specimen of potato in 1846 in
the Royal Botanic Gardens in Dublin, Ireland. It is one of
several of the oldest known specimens of potato that still
exists from potato famine epidemics. The pathogen was then
called Botrytis infestans by British mycologist Miles Berkeley,
and the specimen was part of his herbarium collection.
|
 |
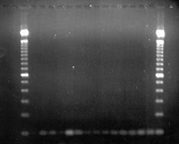 |
DNA
Gel Showing Bands of rDNA for 19th-Century Specimens
North Carolina
State University scientists used molecular methods and a
PCR primer called HERB1 in combination with PINF, a Phytophthora
infestans specific primer, to amplify successfully a 100
pair fragment of the ribosomal DNA for 19th-century dried
potato leaves from herbarium samples. Researchers in the
Ristaino lab have successfully amplified DNA from more than
30 percent of the sampled tested to date which include Irish,
British, and French samples collected in 1845, 1846, and
1847. Molecular studies of herbarium specimens have the
potential to open a new window to study epidemics of the
past.
|
|
|
Dr. Jason
Shih
Keratinase Technology
|
Two
different technologies were invented from Dr. Jason Shih's laboratory.
One derived from the discovery of a feather-degrading bacterium,
which can break down chicken feathers. This discovery is significant,
because feathers, like hair, are made of keratin protein that is
resistant to common digestive enzymes. Because keratin is difficult
to digest, feathers and hair have never been used as dietary protein.
During a series of studies, the enzyme keratinase, which catalyzes
the hydrolysis of feathers, was isolated. The gene that encodes
keratinase was also isolated and sequenced. Cloning of this gene
for hyper-production of this enzyme is now possible. More importantly,
it was demonstrated that feathers after treatment with this enzyme
were indeed hydrolyzed to peptides and amino acids. Feathers processed
with the keratinase, therefore, are convertible to dietary protein
used in animal feeds. The poultry industry in the U.S. produces
eight billion chickens each year. From processing these chickens,
one million tons of feathers are generated each year as a major
by-product, mostly wasted or underutilized. If the keratinase technology
can be adapted to process feathers, it can create a $400 million
market based on the value of digestible protein. The development
of the keratinase technology is a good case study in converting
the waste into a value-added product.
|

|
|
Figure
1: Dr. Jason Shih and his graduate student (now Dr. Scott
Carter) are inspecting the culture of bacteria growing on
feathers. These bacteria produce and secrete the keratinase
enzyme that hydrolyzes feather keratin.
|
| |
| |
| |
| |
| |
| |
| |
|
Pertinent
Patents to Keratinase Technology
|
U.S. Patent Number
|
Title
|
Receiver of Patent and Year Issued
|
|
#4,908,220
|
Feather-Lysate,
A Hydrolyzed Feather Feed Ingredient and Animal Feeds Containing
the Same
|
Jason
C. H. Shih and C. Michael Williams (1990)
|
|
#4,959,311
|
Method
of Degrading Keratinaceous Material and Bacteria Useful Therefor
|
Jason
C. H. Shih and C. Michael Williams (1990)
|
|
#5,063,161
|
Method
of Degrading Keratinaceous Material and Bacteria Useful Therefor,
A Divisional Patent
|
Jason
C. H. Shih and C. Michael Williams (1991)
|
|
#5,171,682
|
Purified
Bacillus licheniformis PWD-1 Enzyme
|
Jason
C. H. Shih and C. Michael Williams (1992)
|
|
#5,186,961
|
Method
and Composition for Maintaining Animals on a Keratin Containing
Diet
|
Jason
C. H. Shih and Chun-Ginn Lee (1993)
|
|
#5,712,147
|
DNA
Encoding Bacillus licheniformis PWD-1 Keratinase
|
Jason
C. H. Shih, Xiang Lin, and Eric S. Miller (1998)
|
|
In addition to
the U.S., these patents have been issued or pending in many foreign
countries, including Canada, Mexico, European Union, South Africa,
Australia, New Zealand, Brazil, China, Taiwan, South Korea, and
Japan.
|
|
Thermophilic
Anaerobic Digestion
U.S.
Patent Number 5,525,229
|
  Click
on images to view
Click
on images to view
|
|
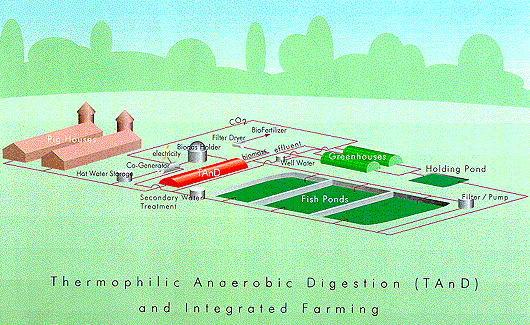
Another
technology invented in Dr. Shih's laboratory is called thermophilic
anaerobic digestion (TAnD). It is a microbiological process by which
organic matter can be degraded and converted to methane and carbon
dioxide in the absence of air or oxygen. The same process can be
used to manage animal waste that is generated in large amount on
a poultry or livestock farm. It was in Dr. Shih's laboratory that
the process was found to be very efficient when operated at higher
(thermophilic) temperatures, first in the laboratory and then on
the university farm. After a successful test with the prototype
at NCSU, the full-scale system was tested successfully in Taiwan
and China. In North Carolina, a full-scale TAnD and associated Integrated
Farming system has been proposed as an alternative method for waste
management on farms. The Integrated Farming system is designed to
fully use all resources or by-products generated by TAnD.
|
|
 |
|
Figure
2: Thermophilic anaerobic digestion (TAnD) and integrated
farming is a new agricultural ecosystem. TAnD converts
animal waste into useful resources including biogas
(65% methane and 35% carbon dioxide) energy, nutrients
for aquaculture and bio-fertilizer for horticultural
produce.
|
|
|
|
|
D
r.
Harold E. Swaisgood
|

|
Process of Removing
the Cooked Flavor from Milk
U.S.
Patent Number 4,053,644
This patent was issued
in 1977 to Dr. Harold E. Swaisgood  of
the food science department at NC State University. Dr. Swaisgood's
invention is a process for removing the "cooked" flavor from milk by
contacting the heat-treated fluid milk with an immobilized sulfhydryl
oxidase enzyme. This method eliminates the unpleasant taste and smell
resembling boiled cabbage that resulted from heating milk in excess
of 155? Fahrenheit. of
the food science department at NC State University. Dr. Swaisgood's
invention is a process for removing the "cooked" flavor from milk by
contacting the heat-treated fluid milk with an immobilized sulfhydryl
oxidase enzyme. This method eliminates the unpleasant taste and smell
resembling boiled cabbage that resulted from heating milk in excess
of 155? Fahrenheit.
|
Purification
and Immobilization of Sulfhydryl Oxidase
U.S.
Patent Number 4,087,328
|
|
|
This
patent was issued in 1978 to Dr. Harold E. Swaisgood of the food
science department at NC State University. This invention is a
process for purification and immobilization of sulfhydryl oxidase
enzyme.
|
|
|
Detection
of Antibiotics in Milk
U.S.
Patent Number 4,347,312
|
  |
|
This patent was
issued in 1982 to Rodney J. Brown of Logan, Utah, and Harold E.
Swaisgood of NC State University's food science department. This
invention is a process for detecting the presence of antibiotics
in milk.
|
Dr.
Kenneth R. Swartzel
Pasteurization and
Aseptic-Packaging for Extended Shelf-Life Liquid Egg
|
Liquid
egg has become extremely important to commercial users of egg because
of its convenience and safety. All liquid egg is pasteurized to
control salmonella, listeria, and other food borne pathogens, as
required by regulatory officials. But egg pasteurization by conventional
methods results in a short shelf-life. The NCSU research team (Hershell
R. Ball, Jr., Mohammad-Hossein Hamid-Samimi, and Kenneth R. Swartzel
developed and patented in 1989, 1990, and 1991 the process technology
that allowed liquid egg products to be marketed free of pathogens
(Listeria as well as Salmonella) and possessing an extended refrigerated
shelf-life. The patents were based upon the equivalent point concept
and a basic study of the rheological properties of egg as a function
of temperature. Until this investigation was completed, pasteurization
temperatures had to be low to avoid egg coagulation. The research
team demonstrated that egg could tolerate higher temperatures than
previously believed, yielding greater destruction of both spoilage
and pathogenic-microorganisms. In follow-up investigations, the
processing technology was perfected and combined with aseptic-packaging
to pave the way for commercialization. The concept used to provide
extended shelf-life egg is now being applied to in-shell pasteurization.
The research efforts of the research team have revolutionized the
liquid egg industry. Morning Glory Egg Company of North Carolina
was quick to recognize the potential of the patents and introduced
Easy Eggs in December 1988. In order to capitalize on the national
market, Michael Foods, Inc., of Minnesota, acquired Morning Glory
and now processes and packages more than 200 million pounds of egg
annually under these patents. In an article about Michael Foods
published in March 1992, Newsweek stated, "Its biggest success is
Easy Eggs, a salmonella (pathogen)-proof liquid egg with a 10-week
shelf-life; sales have risen to $80 million annually since its 1989
introduction." The convenience, safety, and portion-control features
of eggs produced with this process are causing previous shell-egg
users to move to extended shelf-life refrigerated liquid eggs. The
acceptance of the new egg product by major fast food firms (e.g.
Burger King) and institutional users is resulting in a fundamental
change in the structure of how eggs are marketed. The exponential
growth of this product has resulted in a production of nearly 1.5
billion pounds since Easy Eggs were introduced. Egg, without the
shell, offers opportunity for further treatments such as cholesterol
removal. Today, the egg industry has a bright future with increased
international trade possibilities and the potential for providing
consumers with a low-cost, nutritious, stable product that promotes
good health.
|

|
|
Cover
of the September 1994 journal, Food Technology, shows Hershell
R. Ball, Jr. (left) and Kenneth R. Swartzel (right) measuring
the temperature of an ultrapasteurized liquid whole egg product
coming from an aseptic-packaging machine.
|
| |
|
The article details the 1994 IFT Food Technology Industrial
Achievement Award given to Kenneth R. Swartzel, Hershell
R. Ball, Jr., and Mohammad-Hossein Hamid-Samimi of NC State
University. To view in pdf format click: Food
Technology Article
|
| |
| |
| |
| |
|
Pertinent
Patents to Liquid Egg Products
|
U.S.
Patent Number
|
Title
|
Receiver
of Patent and Year Issued
|
|
#4,808,425
|
Method
for the Ultrapasteurization of Liquid Whole Egg Products
|
Kenneth
R. Swartzel, Hershell R. Ball, Jr., and Mohammad-Hossein Hamid-Samimi
(1989)
|
|
#4,957,759
|
Method
for the Ultrapasterurization of Liquid Whole Egg Products
|
Kenneth
R. Swartzel, Hershell R. Ball, Jr., and Mohammad-Hossein Hamid-Samimi
(1990)
|
|
#4,957,760
|
Ultrapasteurization
of Liquid Whole Egg Products with Direct Heat
|
Kenneth
R. Swartzel, Hershell R. Ball, Jr., and Jeffrey W. Liebrecht (1990)
|
|
#4,994,291
|
Method
for the Ultrapasterurization of Liquid Whole Egg
|
Kenneth
R. Swartzel, Hershell R. Ball, Jr., and Mohammad-Hossein Hamid-Samimi
(1991)
|
|
#5,019,407
|
Method
for Pasteurizing Liquid Whole Egg Products
|
Kenneth R. Swartzel
and Hershell R. Ball, Jr. (1991)
|
|
#5,019,408
|
Method
for the Ultrapasteurization of Liquid Whole Egg Products
|
Kenneth
R. Swartzel, Hershell R. Ball, Jr., and Mohammad-Hossein Hamid-Samimi
(1991)
|


 


|


 from videotape produced by the National Science Foundation in 1981.
That
tape explained their recent development and testing of Video Microscopy.
The microscope seen in the picture was a Zeiss Axiomat and Hamamatsu
made the camera and frame memory. They worked with the Hamamatsu engineers
from videotape produced by the National Science Foundation in 1981.
That
tape explained their recent development and testing of Video Microscopy.
The microscope seen in the picture was a Zeiss Axiomat and Hamamatsu
made the camera and frame memory. They worked with the Hamamatsu engineers
 to
modify the video camera and frame memory for this new microscope use.
Robert Allen died from cancer in 1986. Nina Allen moved to Wake Forest
University in 1984 and Joined the Botany Department at
North
Carolina State University in 1995. She is Director of the Cellular and
Molecular Imaging Facility housed in 4115 Gardner Hall. Video microscopy
is used in that laboratory on a daily basis to image biological and
material science objects.
to
modify the video camera and frame memory for this new microscope use.
Robert Allen died from cancer in 1986. Nina Allen moved to Wake Forest
University in 1984 and Joined the Botany Department at
North
Carolina State University in 1995. She is Director of the Cellular and
Molecular Imaging Facility housed in 4115 Gardner Hall. Video microscopy
is used in that laboratory on a daily basis to image biological and
material science objects.